Protein expression services for ETHE1 | Persulfide dioxygenase ETHE1, mitochondrial
Description
Sulfur dioxygenase that plays an essential role in hydrogen sulfide catabolism in the mitochondrial matrix. Hydrogen sulfide (H(2)S) is first oxidized by SQRDL, giving rise to cysteine persulfide residues. ETHE1 consumes molecular oxygen to catalyze the oxidation of the persulfide, once it has been transferred to a thiophilic acceptor, such as glutathione (R-SSH). Plays an important role in metabolic homeostasis in mitochondria by metabolizing hydrogen sulfide and preventing the accumulation of supraphysiological H(2)S levels that have toxic effects, due to the inhibition of cytochrome c oxidase. First described as a protein that can shuttle between the nucleus and the cytoplasm and suppress p53-induced apoptosis by sequestering the transcription factor RELA/NFKB3 in the cytoplasm and preventing its accumulation in the nucleus.
Family
Belongs to the metallo-beta-lactamase superfamily. Glyoxalase II family.
Species
Bos taurus
Length
254 amino acids
Sequence
MAGSVLKIAGRRLSQHTGSGAPVLLRQMFEPKSCTYTYLLGDRESREAVLIDPVLETAQRDAQLVKELGLRLLYAVNTHCHADHITGSGLLRSLLPGCQSVISRLSGAQADWHIEDGDSIQFGRFALETRASPGHTPGCVTFVLNDHSMAFTGDALLIRGCGRTDFQQGCAETLYHSVHEKIFTLPGNCLIYPAHDYHGLTVSTVEEERTLNPRLTLSCEEFVKVMDKLNLPKPQQIDFAVPANMRCGIQTPPS
Mass
27.9 kDa
Simulated SDS-PAGE
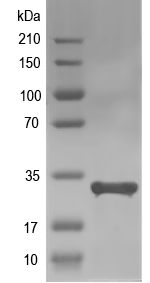 (Note: Representative image - actual molecular weight may vary depending on tag type and expression method)
(Note: Representative image - actual molecular weight may vary depending on tag type and expression method)Safety
Upon ordering, we will perform rigorous biosecurity and export control screening to ensure that order fulfillment is consistent with all legal and regulatory guidance.
Protein synthesis service
Make ETHE1 using our protein expression services starting at $99 + $.30/amino acid in as fast as two weeks (includes the cost of DNA synthesis)
Order Here