Protein expression services for yghU | Disulfide-bond oxidoreductase YghU
Description
Exhibits a robust glutathione (GSH)-dependent disulfide-bond reductase activity toward the model substrate, 2-hydroxyethyl disulfide; the actual physiological substrates are not known. Also displays a modest GSH-dependent peroxidase activity toward several organic hydroperoxides, such as cumene hydroperoxide and linoleic acid 13(S)-hydroperoxide, but does not reduce H(2)O(2) or tert-butyl hydroperoxide at appreciable rates. Exhibits little or no GSH transferase activity with most typical electrophilic substrates, and has no detectable transferase activity toward 1-chloro-2,4-dinitrobenzene (CDNB) with glutathionylspermidine (GspSH) as the nucleophilic substrate.
Family
Belongs to the GST superfamily. Nu-class GSH transferase family.
Species
Escherichia coli (strain K12)
Length
288 amino acids
Sequence
MTDNTYQPAKVWTWDKSAGGAFANINRPVSGPTHEKTLPVGKHPLQLYSLGTPNGQKVTIMLEELLALGVTGAEYDAWLIRIGDGDQFSSGFVEVNPNSKIPALRDHTHNPPIRVFESGSILLYLAEKFGYFLPQDLAKRTETMNWLFWLQGAAPFLGGGFGHFYHYAPVKIEYAINRFTMEAKRLLDVLDKQLAQHKFVAGDEYTIADMAIWPWFGNVVLGGVYDAAEFLDAGSYKHVQRWAKEVGERPAVKRGRIVNRTNGPLNEQLHERHDASDFETNTEDKRQG
Mass
32.4 kDa
Simulated SDS-PAGE
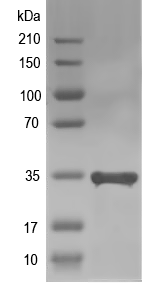 (Note: Representative image - actual molecular weight may vary depending on tag type and expression method)
(Note: Representative image - actual molecular weight may vary depending on tag type and expression method)Safety
Upon ordering, we will perform rigorous biosecurity and export control screening to ensure that order fulfillment is consistent with all legal and regulatory guidance.
Protein synthesis service
Make yghU using our protein expression services starting at $99 + $.30/amino acid in as fast as two weeks (includes the cost of DNA synthesis)
Order Here